
SL Paper 3
A potato chip (crisp) was ignited and the flame was used to heat a test tube containing water.

(i) Calculate the heat required, in kJ, to raise the temperature of the water, using data in the table above and from Table 2 of the Data Booklet.
(ii) Determine the enthalpy of combustion of the potato chip, in \({\text{kJ}}\,{{\text{g}}^{ - 1}}\).
This energy comes mainly from the combustion of triglycerides. State the name of one other type of lipid found in the body and one role, other than energy storage, of this type of lipid.
Name:
Role:
Explain why lipids have a higher energy content than carbohydrates.
Markscheme
(i) \({\text{heat}} = \frac{{4.18 \times 20.0 \times (51.3 - 17.8)}}{{1000}}\);
\( = 2.80{\text{ (kJ)}}\);
(ii) \({\text{enthalpy of combustion}} = \left( {\frac{{2.80}}{{0.421}} = } \right){\text{ }} - 6.65{\text{ (kJ}}\,{{\text{g}}^{ - 1}}{\text{)}}\);
Name:
steroids;
Role:
(sex) hormones;
OR
Name:
phospholipids;
Role:
membranes;
lipids less oxidized/contain less oxygen / carbohydrates partially/more oxidized/contain more oxygen / OWTTE;
Examiners report
This part was generally well answered but there were some cases where 33.5 °C was converted into Kelvin. Many candidates had serious problems with unit conversions and gave the answer as 2800 J or 2800 kJ. Some candidates had correct value for (ii) but lost the mark because of the omission of the negative sign.
Part (b) was well answered.
Very few candidates linked the fact that lipids have higher energy content due to being less oxidized.
Foods such as rice, bread and potatoes are rich in carbohydrates. There are three main types of carbohydrate – monosaccharides, disaccharides and polysaccharides.
Glucose, \({{\text{C}}_{\text{6}}}{{\text{H}}_{{\text{12}}}}{{\text{O}}_{\text{6}}}\), is a monosaccharide. When 0.85 g of glucose was completely combusted in a calorimeter, the temperature of 200.10 g of water increased from 20.20 °C to 27.55 °C. Calculate the energy value of glucose in \({\text{J}}\,{{\text{g}}^{ - 1}}\).
(i) Draw the straight chain structure of glucose.
(ii) Draw the structural formula of \(\alpha \)-glucose.
(iii) Distinguish between the structures of \(\alpha \)- and \(\beta \)-glucose.
(iv) Two \(\alpha \)-glucose molecules condense to form the disaccharide maltose. Deduce the structure of maltose.
One of the major functions of carbohydrates in the human body is as an energy source. State one other function of a carbohydrate.
Markscheme
\(\Delta T = 7.35\) (K/°C);
\(q( = mc\Delta T = {\text{200.10 g}} \times {\text{4.18 J}}\,{{\text{g}}^{ - 1}}{{\text{K}}^{ - 1}} \times {\text{7.35 K}}) = 6.15 \times {10^3}{\text{ J}}\) (per 0.85 g of glucose heated);
energy value \( = 7.2 \times {10^3}{\text{ (J}}\,{{\text{g}}^{ - 1}}{\text{)}}\);
Award [3] for correct final answer.
(i) 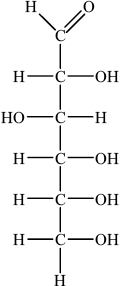
Accept CHO, CH2OH and OH groups on either side of the carbon chain provided OH on C3 is on the opposite side to OHs on C2, C4 and C5.
(ii) 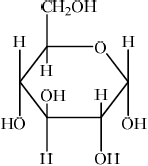 ;
;
Accept CH2OH and OH groups on either face, as long as OH on C3 is on the opposite face to the OH’s on C1, C2 and C4.
No mark awarded if HOCH2 is written, with H bonded to C or if HO is written for hydroxyl groups, with H bonded to C. Penalize this once only in (i), (ii) and (iv).
(iii) the OH on carbon-1/C-1 is inverted / difference in position of OH on carbon-1/C-1;
(iv) 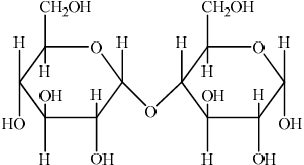 ;
;
energy reserve / can act as precursors in large number of metabolic reactions/for other biologically important molecules;
Examiners report
In (a) SD’s proved the major issue. Most candidates scored the mark for \(\Delta T\). The candidates who struggled made the following errors: incorrect mass (0.85 g) of water was used in \(q = mc\Delta T\), and failure to convert to J per g by dividing \(q\) by 0.85.
In (b)(i) many candidates struggled to draw the straight chain structure of glucose. In many instances the –OH groups were positioned incorrectly and some candidates were careless with bonding writing ‘-C–HO’ rather than ‘-C–OH’.
In (ii) a significant number of candidates mixed up the positions of the substituents on the two faces. In (iii) C1 was commonly omitted. It was surprising to see that quite a few candidates could not draw the structure of maltose in (iv). The most common mistake involved an incorrect linkage
Part (c) was answered well by the vast majority.
Granola bars are a source of dietary fibre.
When 1.13 g of a granola bar was combusted in a bomb calorimeter, the temperature of \({\text{500 c}}{{\text{m}}^{\text{3}}}\) of water increased from 18.5 °C to 28.0 °C. Calculate the energy value, in kJ per 100 g, of the granola bar to the correct number of significant figures.
Markscheme
energy (released by granola) \( = (500 \times 4.18 \times 9.5 = ){\text{ }}19855{\text{ (J)}}/2.0 \times {10^4}{\text{ (J)}}\);
energy released (in kJ per 100 g) \(\left( {\frac{{19855}}{{1000}} \times \frac{{100}}{{1.13}} = } \right){\text{ }}1757.079\);
\( = 1.8 \times {10^3}{\text{ (kJ per 100 g)}}\);
Allow 1800 (kJ per 100 g).
Award [3] for correct final answer.
Award [2 max] for 1760 (kJ per 100 g).
Remember to allow ECF.
Examiners report
Candidates generally used the appropriate equations to solve for the energy value for part (a) however, many included 1.13g with the mass of water and on quite a few occasions, \({\text{22.4 d}}{{\text{m}}^{\text{3}}}\) was used in the question for to calculate energy per gram. Several missed the final mark for not expressing the final value in 2 significant figures. Candidates were not able to apply the rules for addition and subtraction within the mathematical process. Surprisingly defining dietary fibre in part (b) scored poorly. Many omitted reference to plant material or cellulose; there appears to be a general misunderstanding that any food that is not digested is dietary fibre. Most candidates were able to name two health problems for (b)(ii).
Glucose, C6H12O6, is a monosaccharide that our body can use as a source of energy.
Deduce the equation for the cellular respiration of glucose.
Calculate the energy, in kJ, produced from 15.0g of glucose if its enthalpy of combustion is −2803kJmol−1.
Glucose is the basic building block of starch which can be used to make bioplastics. Outline two advantages and two disadvantages of biodegradable plastics.
Two advantages:
Two disadvantages:
Bioplastics are broken down by enzyme catalysed reactions. Sketch a graph illustrating how the rate of this reaction varies with pH.
Markscheme
C6H12O6 (aq) + 6O2 (aq) → 6CO2 (aq) + 6H2O (l)
Accept equations for anaerobic respiration, such as C6H12O6 (aq) → 2C3H6O3 (aq)
Ignore ATP if added as a product.
\(n\left( {{{\rm{C}}_{\rm{6}}}{{\rm{H}}_{{\rm{12}}}}{{\rm{O}}_{\rm{6}}}} \right)\left\langle { = \frac{{15.0}}{{180.18}}} \right\rangle = 0.0833 \ll {\rm{mol}} \gg \)
«energy=0.0833×2803=»233«kJ»
Award [2] for correct final answer.
Accept -233«kJ».
Two advantages:
renewable resource
broken down/digested by bacteria or other organisms within a relatively short time/quickly
reduce «volume of» plastic waste/landfill
reduce use of petrochemicals
OR
reduce use of fossil fuels as hydrocarbon source
degrade into non-toxic products
Any two advantages for [2 max].
M2: reference must be made to time. Do not accept “biodegradable” (since stated in question).
Ignore any mention of cost.
Two disadvantages:
require use of land «for crop production»
increased use of fertilizers/pesticides «leading to pollution»
OR
eutrophication
might break down before end of use
release of methane/CH4/greenhouse gas «during degradation»
Any two disadvantages for [2 max].
Ignore any mention of cost.
typical curve as shown in example above √
Accept any curve with a single maximum (not just bell-shaped).
Ignore features such as pH values on a pH scale or a pH value at maximum (if given).
Do not penalize if curve does not touch the x-axis.
Examiners report
Vegetable oils, such as that shown, require conversion to biodiesel for use in current internal combustion engines.
State two reagents required to convert vegetable oil to biodiesel.
Deduce the formula of the biodiesel formed when the vegetable oil shown is reacted with the reagents in (a).
Explain, in terms of the molecular structure, the critical difference in properties that makes biodiesel a more suitable liquid fuel than vegetable oil.
Determine the specific energy, in kJ\(\,\)g−1, and energy density, in kJ\(\,\)cm−3, of a particular biodiesel using the following data and section 1 of the data booklet.
Density = 0.850 g\(\,\)cm−3; Molar mass = 299 g\(\,\)mol−1;
Enthalpy of combustion = 12.0 MJ\(\,\)mol−1.
Markscheme
methanol
OR
ethanol
strong acid
OR
strong base
Accept “alcohol”.
Accept any specific strong acid or strong base other than HNO3/nitric acid.
[3 marks]
CH3(CH2)16COOCH3 / CH3OCO(CH2)16CH3
OR
CH3(CH2)16COOC2H5 / C2H5OCO(CH2)16CH3
Product must correspond to alcohol chosen in (a), but award mark for either structure if neither given for (a).
[1 mark]
lower viscosity
weaker intermolecular/dispersion/London/van der Waals’ forces
OR
smaller/shorter molecules
Accept “lower molecular mass/Mr” or “lower number of electrons”.
Accept converse arguments.
[2 marks]
Specific energy: «\( = \frac{{12\,000{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}}}{{299{\text{ g}}\,{\text{mo}}{{\text{l}}^{ - 1}}}}\)» = 40.1 «kJ g−1»
Energy density: «= 40.1 kJ\(\,\)g−1 x 0.850 g\(\,\)cm−3» = 34.1 «kJ\(\,\)cm−3»
Award [1] if both are in terms of a unit other than kJ (such as J or MJ).
[2 marks]
Examiners report
Polymers are made up of repeating monomer units which can be manipulated in various ways to give structures with desired properties.
(i) Draw the structure of 2-methylpropene.
(ii) Deduce the repeating unit of poly(2-methylpropene).
Deduce the percentage atom economy for polymerization of 2-methylpropene.
(i) Suggest why incomplete combustion of plastic, such as polyvinyl chloride, is common in industrial and house fires.
(ii) Phthalate plasticizers such as DEHP, shown below, are frequently used in polyvinyl chloride.
With reference to bonding, suggest a reason why many adults have measurable levels of phthalates in their bodies.
Markscheme
i
OR
H2C=C(CH3)2
ii
OR
−CH2C(CH3)2−
Continuation bonds needed for mark.
No penalty if square brackets present or “n” appears after the bracket/formula.
«same mass of product as reactant, thus» 100«%»
Accept “less than 100%” only if a reason is given (eg, the catalyst is not converted into the product, or other reasonable answer).
i
due to stability of plastics/strong covalent bonds
OR
low volatility preventing good mixing with oxygen «gas»
OR
lack of/insufficient oxygen
OR
plastics are often parts of devices with non-combustible components «which mechanically prevent the combustion of plastic components»
OR
PVC already partly oxidised «because some C–H bonds are replaced with C–Cl bonds», so it cannot produce enough heat for complete combustion
OR
many industrial/household materials contain additives that reduce their flammability/act as flame retardants
ii
weakly bound to the PVC/no covalent bonds to PVC/only London/dispersion/instantaneous induced dipole-induced dipole forces between DEHP and PVC AND leach/evaporate «from PVC» to atmosphere/food chain
OR
has low polarity/contains non-polar hydrocarbon chains AND fat-soluble/deposits in the fatty tissues
OR
has unusual structural fragments/is a xenobiotic/difficult to metabolise AND stays in the body for a long time
Examiners report
A class was determining the concentration of aqueous sodium hydroxide by titrating it with hydrochloric acid, whilst monitoring the pH of the solution. The sodium hydroxide solution was added into a glass beaker from a measuring cylinder and the hydrochloric acid added using a burette. One group of students accidentally used a temperature probe rather than a pH probe. Their results are given below.
Volume of aqueous NaOH = 25.0 ± 0.5 cm3
Concentration of HCl = 1.00 ± 0.01 mol dm−3
The graph of temperature against titre can be used to calculate the concentration of alkali without knowing the concentration of the hydrochloric acid, using the enthalpy of neutralization.
Explain how the concentration may be calculated in this way.
Heat losses would make this method less accurate than the pH probe method. Outline why the thermometric method would always give a lower, not a higher, concentration.
Suggest how heat loss could be reduced.
State one other assumption that is usually made in the calculation of the heat produced.
Suggest why scientists often make assumptions that do not correspond to reality.
Outline why the thermochemical method would not be appropriate for 0.001 mol\(\,\)dm−3 hydrochloric acid and aqueous sodium hydroxide of a similar concentration.
Markscheme
heat change/evolved can be calculated from the «maximum» temperature increase and the mass of solution
OR
q = mcΔT
heat «evolved» gives the number of moles «of both acid and alkali present when neutralisation occurs»
OR
\(n = \frac{q}{{\Delta {H_{neut}}}}\)
volume «of acid and the volume of alkali required to just neutralise each other» can be used to calculate the concentration«s of both»
OR
\(\left[ {{\text{NaOH}}} \right] = \frac{n}{V}\)
[2 marks]
smaller temperature increase/ΔT
OR
heat released would «appear to» be less
amount of substance/n calculated is smaller
[2 marks]
using «expanded» polystyrene cup
OR
insulating beaker
OR
putting a lid on beaker
Do not accept calorimeter by itself.
Accept any other reasonable suggestion.
[1 mark]
«specific» heat capacity of the beaker/container/thermometer is ignored
OR
density of the solutions is assumed as 1.00 g\(\,\)cm–3/same as water
OR
specific heat capacity of the solutions is assumed as 4.18 J g–1\(\,\)K–1/same as water
Accept “reaction goes to completion”.
Accept “reaction is conducted under standard conditions”.
Accept “no evaporation occurs”.
Accept any other relevant valid assumption.
Do not accept “heat is not released from other reactions”.
[1 mark]
allows simple theories to be applied to real life situations
OR
enables us to start to understand complex situations
OR
gives answers that are accurate to the required order of magnitude
OR
simplifies the calculations involved
Do not accept “to simplify the situation” without further detail.
Accept “errors do not have a major impact on the results”.
[1 mark]
temperature rise would be too small «to be accurately measured»
Accept “heat released would be too small «to be accurately measured»”.
[1 mark]